Introduction
Laser absorption is the starting point of all subsequent laser–matter interactions. How a material “accepts” laser energy determines whether it undergoes melting, vaporization, or more complex physicochemical reactions. Understanding this initial energy coupling process is essential for optimizing processing and solving technical challenges.
Absorption is not a single mechanism but the result of multiple intertwined processes. These mechanisms are highly sensitive to laser parameters (wavelength, intensity, pulse duration) and to material properties (metal, semiconductor, glass). This article explores the principles and mechanisms of laser energy absorption:
- How do laser properties enable efficient absorption?
- How do electrons in metals, semiconductors, and insulators capture laser energy before plasma forms?
- Which factors influence absorption efficiency?
- How is absorbed energy dissipated (heating, bond breaking, shock)?
- What are the macroscopic results (melting, vaporization, ablation, modification)?
Key Laser Properties
Laser–matter interaction is defined by the unique optical properties of lasers, which govern whether and how absorption occurs, and what results follow.
Monochromaticity
Lasers emit light at a single wavelength. Since material absorption spectra strongly depend on wavelength, monochromaticity allows selective excitation of specific electronic or vibrational transitions—provided the wavelength matches the absorption peak.
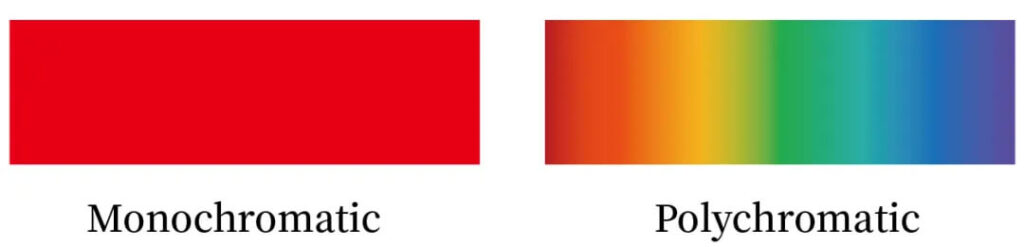
Figure 1:Monochromatic vs. polychromatic light
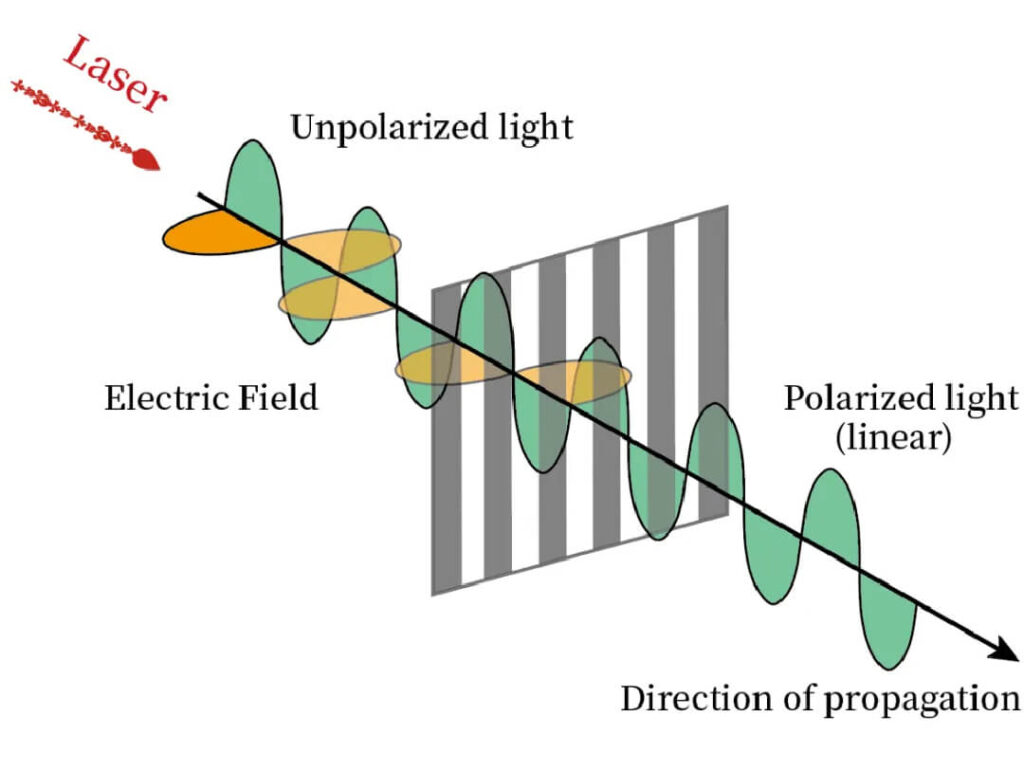
Coherence
Lasers exhibit high phase coherence. Spatial coherence enables focusing to diffraction-limited spots, reaching extremely high intensities.
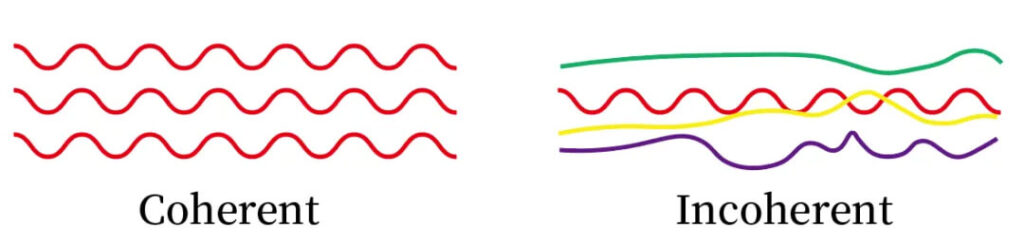
Figure 2:Laser vs. ordinary light coherence
Intensity
High intensity (power per unit area) drives nonlinear absorption processes such as multiphoton absorption, even in normally transparent materials.
Pulse Duration
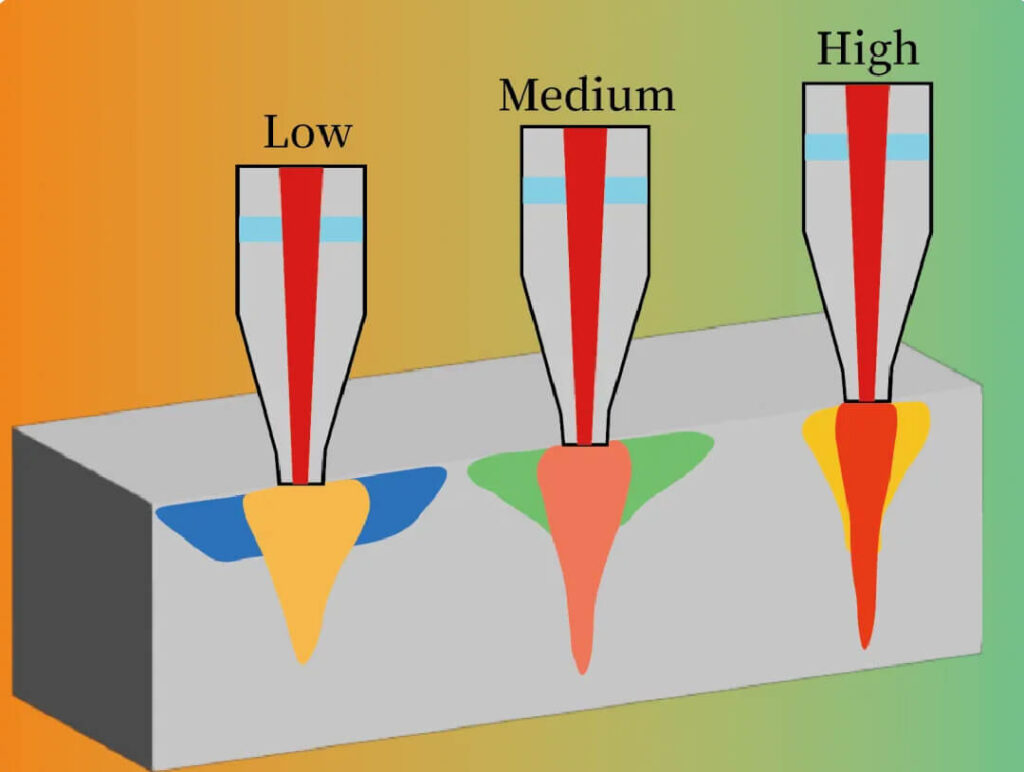
Pulse width defines the rate of energy delivery. Ultrashort pulses (fs, ps) can deposit energy before thermal relaxation occurs, leading to nonthermal and nonequilibrium effects.
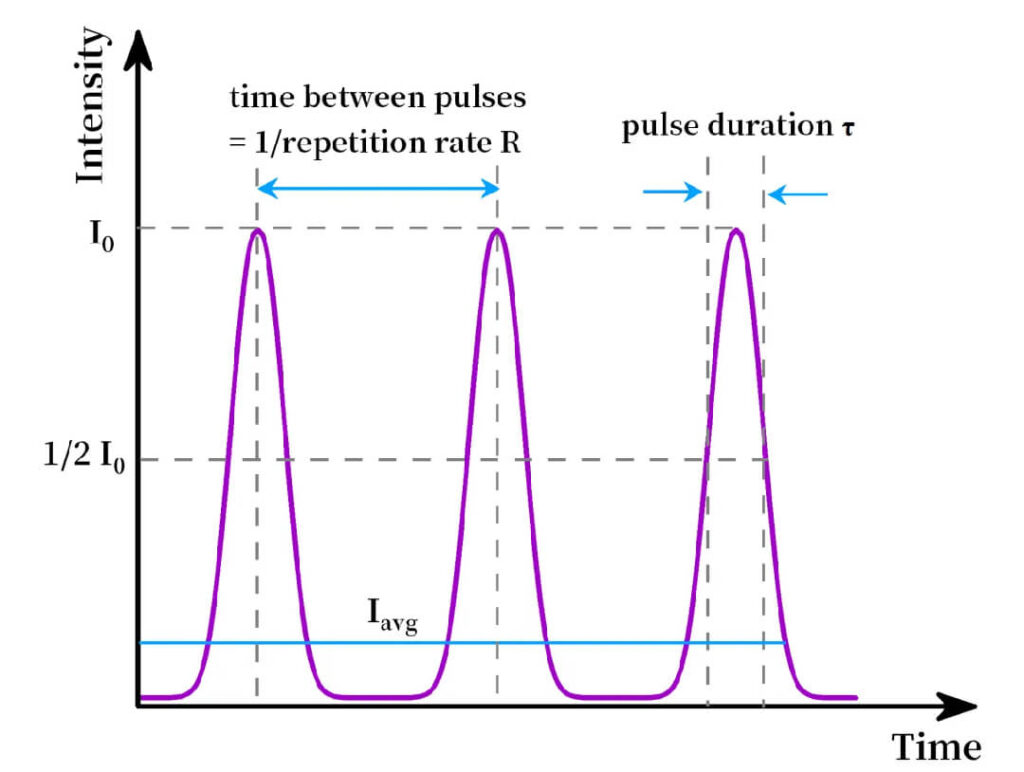
Figure 4:Pulse duration comparison
Polarization
The oscillation direction of the laser’s electric field affects absorption, especially in anisotropic materials or at special incidence angles (e.g., Brewster’s angle).
Fundamental Absorption Mechanisms
Before plasma forms, energy is absorbed by electrons according to the material’s electronic structure.
Metals: Free-Electron Absorption (Drude Model)
Conduction electrons respond to the oscillating electric field, then transfer energy to the lattice through electron–phonon scattering, heating the material.
- Intra-band transitions: electrons absorb photons within the same band.
- Inter-band transitions: higher-energy photons excite electrons across bands.
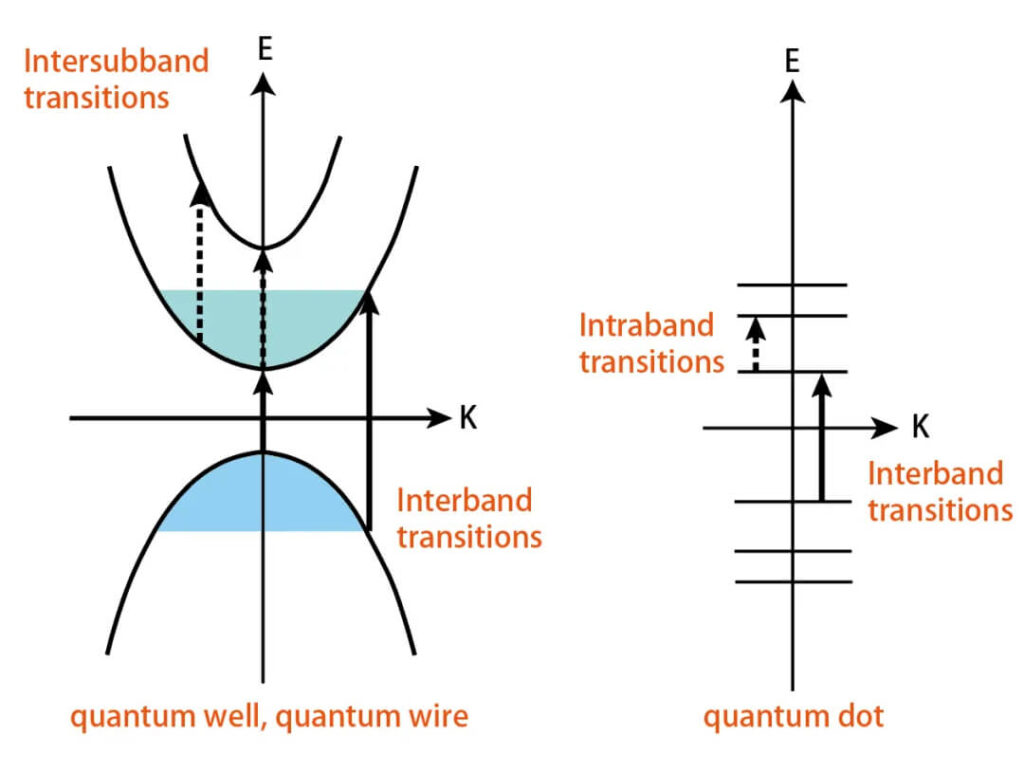
Figure 6:Intra- vs. inter-band transitions
Even though metals reflect strongly, residual energy is absorbed within the shallow skin depth (tens of nm).
Semiconductors and Dielectrics: Bandgap Effects
The bandgap EgE_g separates filled valence bands from empty conduction bands.
- Direct absorption (ℏω>Eg\hbar\omega > E_g): electrons jump directly to the conduction band, forming electron-hole pairs.
- Nonlinear absorption (ℏω<Eg\hbar\omega < E_g):
Multiphoton absorption (MPA): multiple low-energy photons excite an electron across the bandgap.
Avalanche ionization: seed electrons, once accelerated, collide and generate more carriers, exponentially increasing absorption.
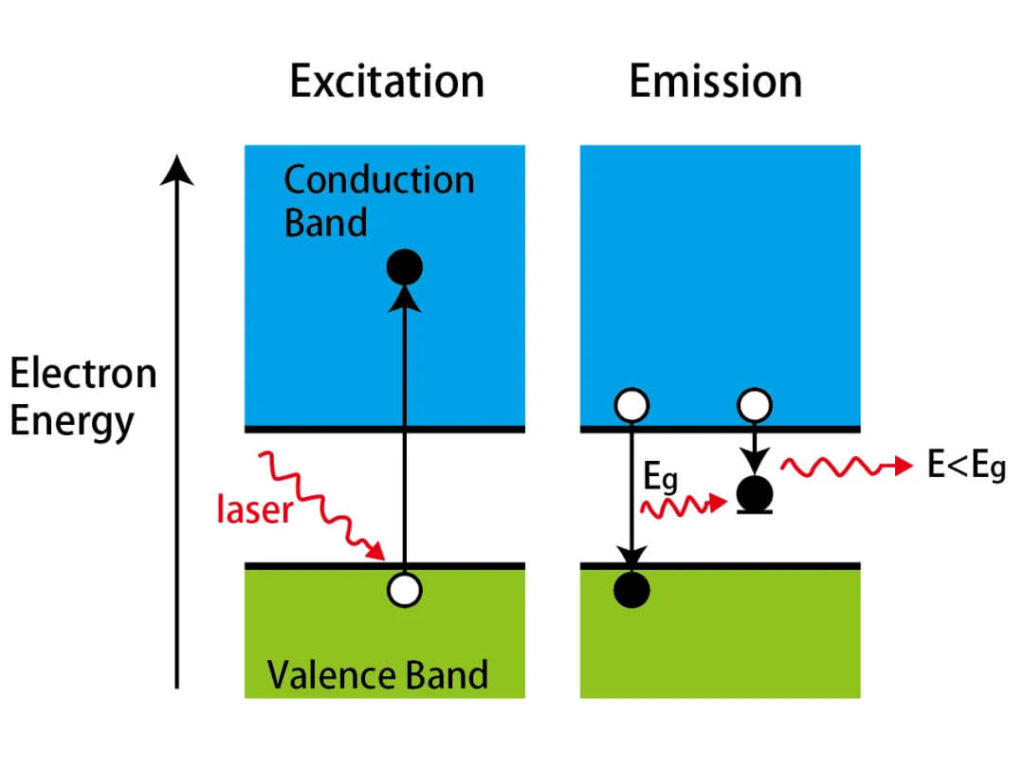
Figures 7–8:Bandgap absorption and avalanche ionization
Key Factors Affecting Absorption
Laser and material parameters jointly determine efficiency.
Laser Parameters
- Wavelength (λ\lambda): defines photon energy and must match material absorption properties.
- Intensity / Fluence: governs nonlinear absorption and overall energy deposition.
- Pulse Duration (τp\tau_p): separates “cold processing” (fs, ps) from thermal effects (ns).
- Polarization: influences anisotropic absorption.
Material Parameters
- Electronic structure (bandgap EgE_g): fundamental determinant of absorption spectrum.
- Optical constants (n, k, R): define how much energy enters and is absorbed.
- Temperature and phase: melting or heating alters reflectivity and absorption coefficients.
- Surface morphology: rough surfaces enhance absorption via multiple reflections.
Absorption itself modifies material properties in real time, creating dynamic feedback during laser–matter interaction.
Laser-Induced Plasma
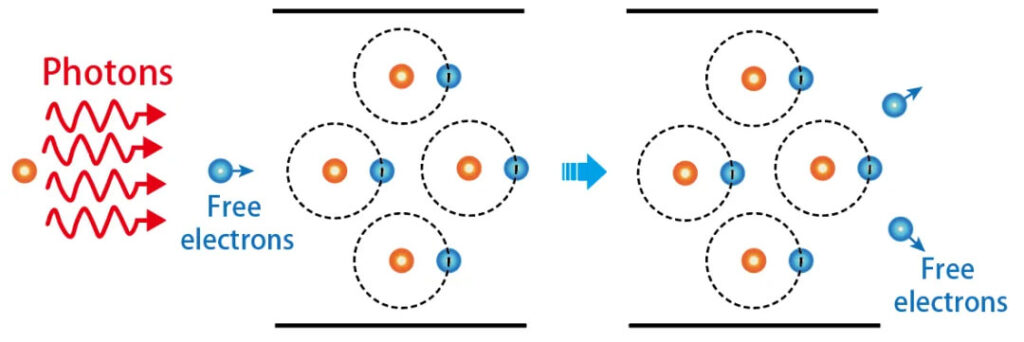
Once deposited energy is sufficient, the material becomes plasma—a mix of electrons, ions, and neutrals.
- Multiphoton ionization (MPI): multiple photons ionize atoms directly.
- Avalanche ionization: electrons gain enough energy to ionize others, rapidly multiplying carriers.
Plasma Absorption Mechanisms
- Inverse Bremsstrahlung (IB): free electrons absorb photons during collisions, heating the plasma.
- Resonance absorption: laser field drives plasma waves, efficiently transferring energy.
Plasma Shielding
At critical density, plasma reflects incoming light, blocking energy transfer. This dual role—efficient absorption vs. shielding—poses challenges for process control.
Relaxation Pathways
Absorbed energy redistributes via electron and lattice interactions.
Two-Temperature Model (TTM)
- Photon absorption (fs): electrons rapidly heat (Te >> Tl).
- Electron–electron scattering (50–100 fs): hot electrons thermalize.
- Electron–phonon coupling (0.1–10 ps): energy flows to lattice.
- Phonon–phonon interactions (>10 ps): energy spreads via heat conduction.
Pulse duration relative to these timescales determines whether nonequilibrium or thermal effects dominate.
Macroscopic Outcomes
The final effect depends on energy delivery rate and fluence:
- Photothermal effects: heating, melting, vaporization (common for long pulses, CW lasers).
- Photochemical effects: bond breaking or reactions triggered by photon energy.
- Photomechanical effects: rapid localized expansion creates stress waves or shock, leading to ablation or ejection (dominant in ultrashort pulses).
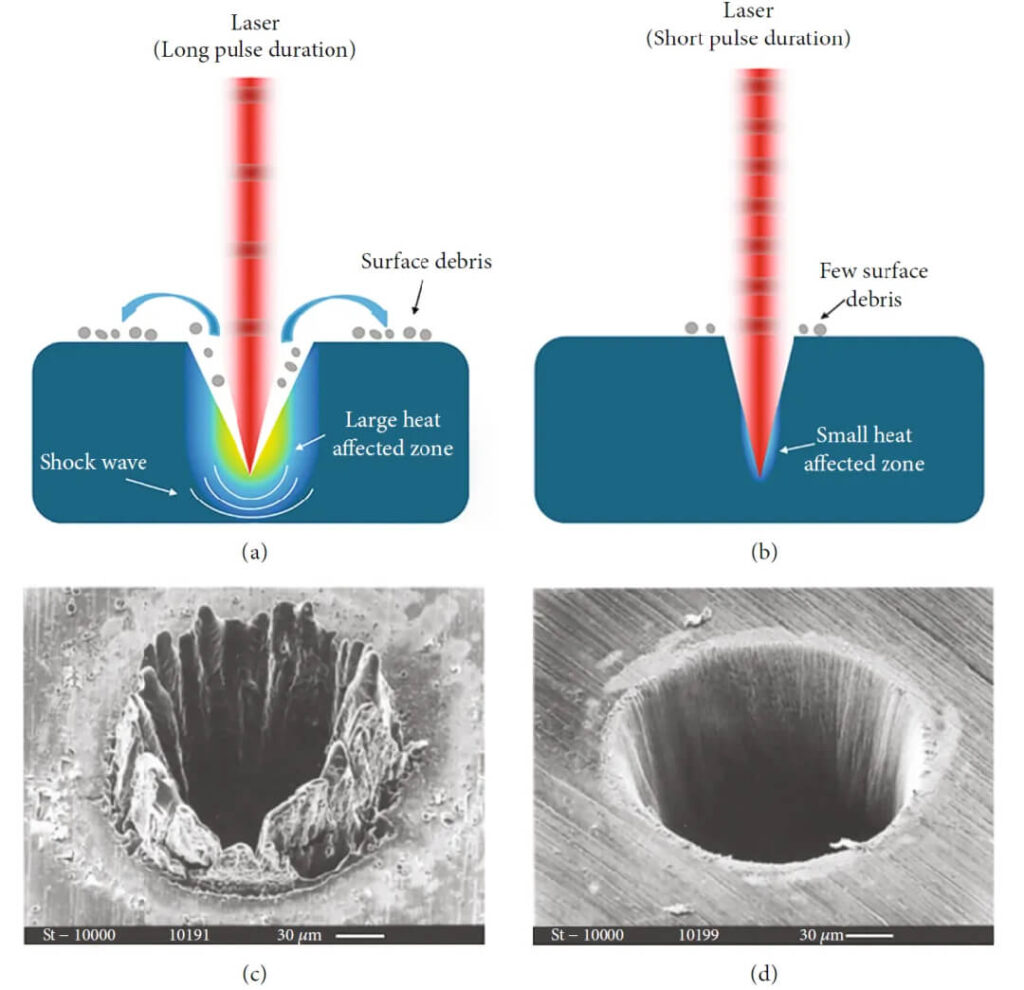
Figure 9:Long vs. short pulse effects
References:
1.Brandau, B.; Da Silva, A.; Wilsnack, C.; Brueckner, F.; Kaplan, A.F.H. Absorbance Study of Powder Conditions for Laser Additive Manufacturing. Mater. Des.2022, 216, 110591, doi:10.1016/j.matdes.2022.110591.
2.Wang, S.; Yang, J.; Deng, G.; Zhou, S. Femtosecond Laser Direct Writing of Flexible Electronic Devices: A Mini Review. Materials 2024, 17, 557. https://doi.org/10.3390/ma17030557.
3.Kavokine, N.; Zou, S.; Liu, R.; Niguès, A.; Zou, B.; Bocquet, L. Ultrafast Photomechanical Transduction through Thermophoretic Implosion. Nat. Commun.2020, 11, 50, doi:10.1038/s41467-019-13912-w.
4.Silaeva, E.; Saddier, L.; Colombier, J.-P. Drude-Lorentz Model for Optical Properties of Photoexcited Transition Metals under Electron-Phonon Nonequilibrium. Appl. Sci. 2021, 11, 9902. https://doi.org/10.3390/app11219902